Aggregated News
A medicine built around a more precise form of CRISPR gene editing appeared to work as designed in its first clinical trial test, developer Beam Therapeutics said Tuesday. But the death of a trial participant could renew concerns about an older drug used alongside Beam’s genetic medicine.
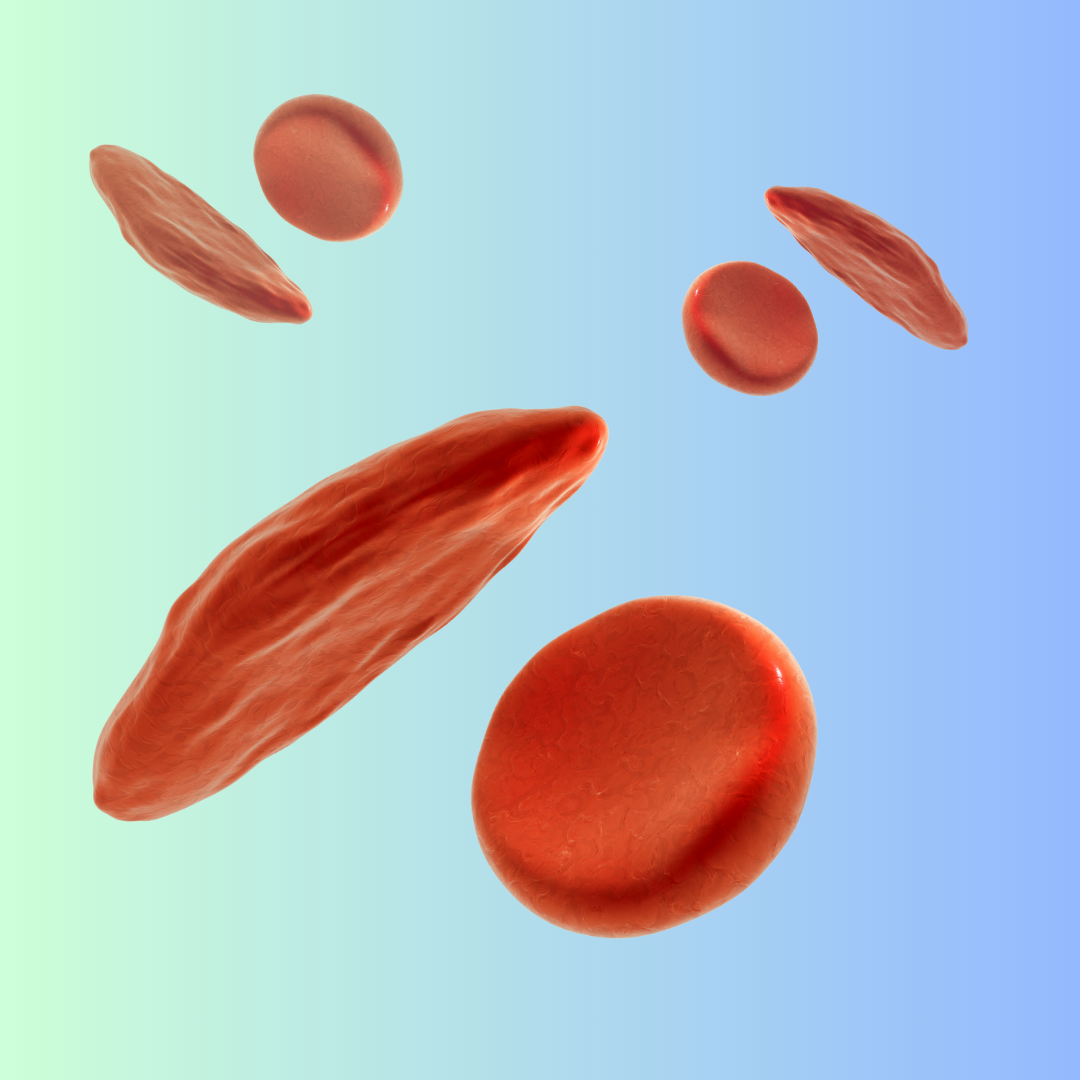
Beam’s medicine uses a technology known as base editing to activate a gene in stem cells collected from people with sickle cell disease, an inherited blood condition that can cause debilitating pain and a constellation of other symptoms.
Data shared by Beam from the first handful of patients treated in the trial show the company successfully edited those cells in a laboratory. When later reinfused back into patients’ bodies, they matured into red blood cells that were more durable and less likely to warp into the sharp-edged crescents associated with the disease.
However, one of the patients died from lung damage that was judged by their physician and the trial’s monitoring committee as related to an old chemotherapy drug commonly used prior to stem cell transplants. The Food and Drug Administration also reviewed the...



