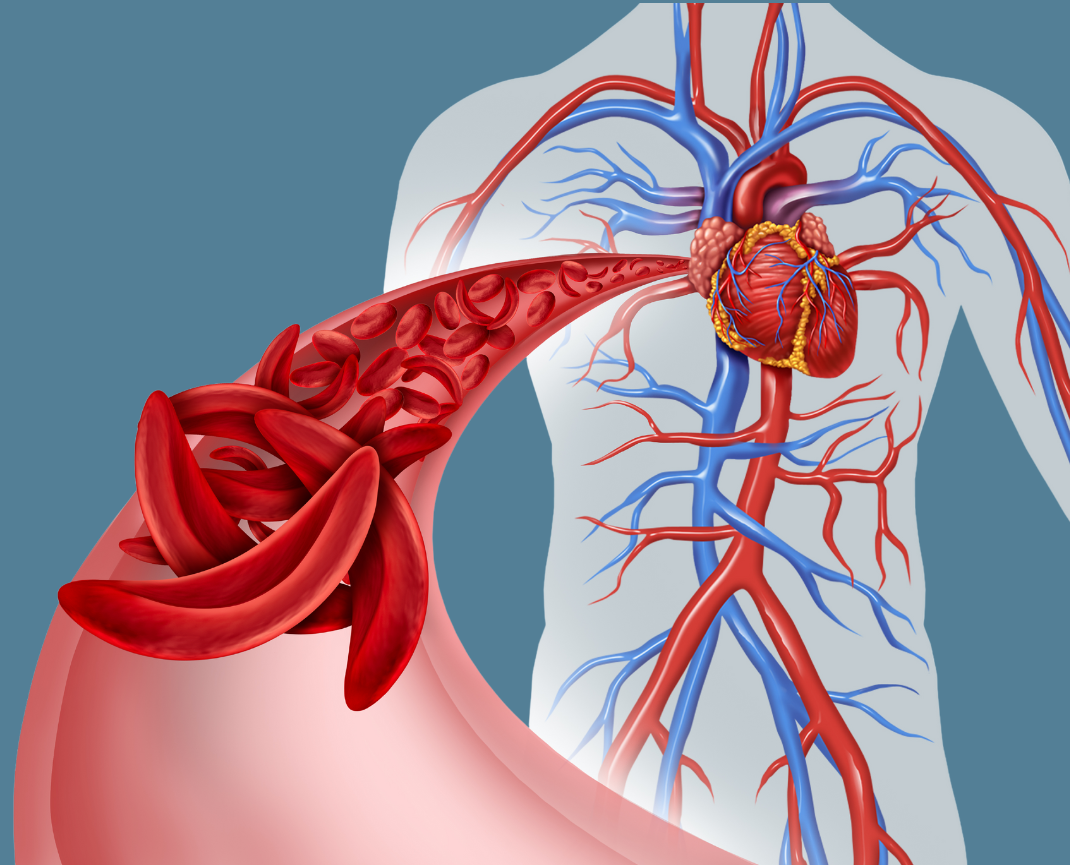
New way for states to cover pricey gene therapies will start with sickle cell disease
By Nada Hassanein,
New Jersey Monitor
| 03. 14. 2024
The U.S. Food and Drug Administration late last year approved two breakthrough gene therapies for sickle cell disease patients. Now a new federal program seeks to make these life-changing treatments available to patients with low incomes — and it could be a model to help states pay for other expensive therapies.
The new sickle cell treatments have brought hope to those with the debilitating blood disorder, which is hereditary and disproportionately affects Black people. But the therapies come with a price tag of as much as $3 million for a course of treatment, which can take up to a year. Despite those high upfront costs, cell and gene therapies have the potential to reduce health care spending over time by addressing the underlying cause of the disease.
Under the so-called Cell and Gene Therapy Access Model, the federal government will negotiate discounts with sickle cell drug manufacturers Vertex Pharmaceuticals, CRISPR Therapeutics and Bluebird Bio on behalf of state Medicaid agencies, which provide health care coverage to low-income patients. To participate, state Medicaid agencies must agree to prices based on those negotiations...
Related Articles
By Liyan Qi and Jonathan Cheng, The Wall Street Journal | 03.26.2025
photo via Wikimedia Commons licensed under CC by 3.0
Chinese scientist He Jiankui set off global outrage and landed in prison after he skirted ethical guidelines and claimed he had produced genetically modified babies designed to resist HIV infection.
Now, the self-styled ...
By Anna Louie Sussman, The New York Times | 03.25.2025
On June 24, 2022, the same day the Supreme Court issued its decision in Dobbs v. Jackson Women’s Health Organization, I received a call from the fertility clinic where I’d been undergoing in vitro fertilization, informing me that seven of...
By Michael Gibney, PharmaVoice | 03.20.2025
The death this week of a teenager receiving Sarepta Therapeutics’ gene therapy Elevidys for Duchenne muscular dystrophy is a tragic reminder of the stakes involved in cutting-edge biotech innovation.
While gene therapies like Sarepta’s offer an opportunity to treat and...
By Staff, The Medicine Maker | 03.21.2025
"The Promise and Peril of CRISPR" cover by Johns Hopkins University Press
As a paediatrician taking care of children with sickle cell disease, Neal Baer, a Harvard Medical School graduate, was in awe of the power of CRISPR technologies. Later...